What is Chemical Nomenclature?
This is a set of rules to generate systematic names for compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry (IUPAC).
The Algorithm
Compound contains Metal
Type I - Non-Transitive Metal
For compounds whose metal is not a transitive metal.
Ionic Binary
These are bonds between a non-transitive metal and a nonmetal.
The name generated for these type of bonds follow this format:
Ionic Ternary
These are bonds between a non-transitive metal and a polyatomic ion.
Polyatomic ions are ions ( charged substances ) that are composed of two or more elements.
The name generated for these types of bonds follows this format:
For the names of the polyatomic ions, refer to the guide at the end or click here.
Type II - Transitive Metal
For compounds whose metal is a transitive metal. Compounds under this type have a Stock/Systematic name and a Common/Classical name.
Ionic Binary
These are bonds between a transitive metal and a nonmetal.
The name generated for these type of bonds follow the following formats
| Stock/Systematic | Common/Classical |
|---|---|
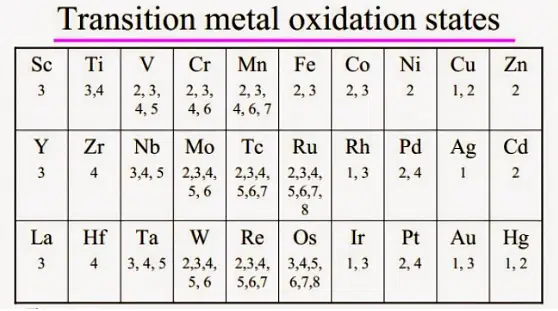
Note: the “-ous” suffix is used to refer to the lesser oxidation state ( ) of a given element; and the “-ic” suffix is used to refer to the greater oxidation state. For a guide on the oxidation states, click here.
Ionic Ternary
These are bonds between a transitive metal and a polyatomic ion.
The name generated for these type of bonds follow the following formats:
| Stock/Systematic | Common/Classical |
|---|---|
Compounds Not Containing Metals
Type III - No Leading Hydrogen
These are molecules or compounds that do not contain a leading hydrogen in their chemical formulas.
Diatomic Molecules
These are molecules composed of 2 atoms of the same elements.
They are referred to only by their element names.
The diatomic atoms are:
- Flourine ( )
- Chlorine ( )
- Bromine ( )
- Iodine ( )
- Nitrogen ( )
- Hydrogen ( )
- Oxygen ( )
Covalent Binary
These are compounds composed of two nonmetals bonded via a covalent bond.
The names generated for these follow this format: The prefixes to be used are the following:
| Subscript | Prefix |
|---|---|
| 1 | mono- |
| 2 | di- |
| 3 | tri- |
| 4 | tetra- |
| 5 | penta- |
| 6 | hexa- |
| 7 | hepta- |
| 8 | octa- |
| 9 | nona- |
| 10 | deca- |
Note: When the first letter of the element is a vowel, the last letter of the prefix can be omitted (Ex. ).
Note: When the first element has a subscript of 1, a prefix of “mono-” is not necessary.
Acid
These are elements that contain a leading hydrogen atom in their chemical formula.
Hydrogen + Element
These are bonds between an hydrogen atom and a nonmetal.
The name generated for these type of bonds follow this format:
Hydrogen + Polyatomic Ion
These are bonds between an hydrogen atom and a polyatomic ion.
The name generated for these type of bonds follow the following formats:
| Case | Format |
|---|---|
| Polyatomic Ion ending in “-ate” | |
| Polyatomic Ion ending in “-ite” |
Polyatomic Ions
- Acetate
- or
- Ammonium
- Arsenate
- Arsenite
- Azide
- Borate
- Bromate
- Bromite
- Carbonate
- Chlorate
- Chlorite
- Chromate
- Chromite
- Citrate
- Cyanate
- Cyanide
- Dichromate
- Dihydrogen Phosphate
- Dihydrogen Phosphite
- Dithionate
- Dithionite
- Ferricyanide
- Ferrocyanide
- Fulminate
- Hydrazide
- Hydrogen Arsenate
- Hydrogen Carbonate
- Hydrogen Phosphate
- Hydrogen Phospite
- Hydrogen Sulfate
- Hydrogen Sulfite
- Hydroxide
- Hypobromite
- Hypochlorite
- Hypoiodite
- Iodate
- Iodite
- Isocyanate
- Manganate
- Nitrate
- Nitrite
- Oxalate
- Ozonide
- Perbromate
- Perchlorate
- Periodate
- Permanganate
- Peroxide
- Perrhenate
- Phosphate
- Phosphite
- Plumbate
- Plumbite
- Rhenate
- Selenate
- Selenite
- Silicate
- Stannate
- Sulfate
- Sulfite
- Superoxide
- Thiosulfate
- Tungstate
Oxidation States
Representative Elements
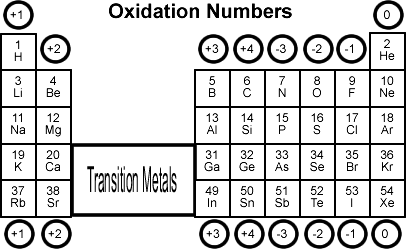
Note: Group 4A or the Crystallogens can either gain 4 electrons ( ) or lose 4 electrons ( ).
Transition Elements